Abstract

Introduction: β-thalassemia is characterized by ineffective erythropoiesis and subsequent anemia of varying severity. Patients with non-transfusion dependent β-thalassemia (NTDT) have historically remained transfusion-independent due to the perception that their anemia is mild to moderate. However, recent evidence has consistently highlighted that in patients with NTDT, hemoglobin (Hb) levels < 10 g/dL are associated with greater long-term morbidity and mortality, with increases of 1 g/dL associated with significant risk reduction. Apart from red blood cell transfusions (RBCTs) administered in certain clinical situations, there is no approved treatment for NTDT-related anemia. RBCTs may provide symptom relief but are associated with adverse events such as iron overload and their own treatment burden related to transfusion requirements. The randomized, double-blind, placebo-controlled phase 2 BEYOND trial (NCT03342404) demonstrated the efficacy and safety of luspatercept, a first-in-class erythroid maturation agent, in patients with NTDT and Hb ≤ 10 g/dL. The study met the primary endpoint with 77.1% and 0% of patients in the luspatercept and placebo groups, respectively, achieving ≥ 1.0 g/dL increase from baseline in mean Hb. This post hoc analysis evaluated the proportion of patients receiving luspatercept who transitioned to Hb thresholds with lower morbidity/mortality risk and who achieved clinically relevant Hb increases across different baseline Hb subgroups.
Methods: The methods of the BEYOND trial have been reported previously (Taher AT, et al. HemaSphere 2021;5(suppl 2). EHA Abstract S101). Briefly, 145 adults (≥ 18 years) with non-transfusion dependent (≤ 5 RBC units [1 unit = 200-350 mL transfused packed RBCs]/24 weeks, and RBCT-free > 8 weeks before randomization) β-thalassemia or HbE/β-thalassemia and Hb ≤ 10.0 g/dL were randomized 2:1 to luspatercept 1 mg/kg (up to 1.25 mg/kg; n = 96) or placebo (n = 49) subcutaneously every 3 weeks for ≥ 48 weeks. All patients continued receiving best supportive care, including sporadic RBCTs and iron chelation therapy. This analysis evaluated the proportion of patients receiving luspatercept who achieved Hb > 10.0 g/dL at Weeks 12, 24, 36, and 48, and the proportion of patients treated with luspatercept who achieved a mean Hb increase ≥ 1.0 g/dL during any rolling 24-week period by baseline Hb level (< 7.0 g/dL, ≥ 7.0 and ≤ 8.5 g/dL, and > 8.5 g/dL). All findings were analyzed descriptively.
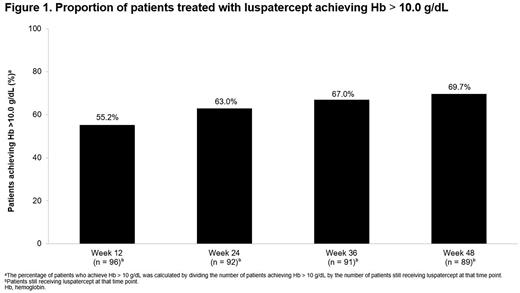
Results: A total of 96 patients receiving luspatercept in the BEYOND intention-to-treat population were eligible and included in the analysis. Median age was 39.5 years (range, 18.0-71.0) and 58.3% of patients were female. At baseline, the median Hb level was 8.2 g/dL (range, 5.3-10.1). Increasing proportions of patients treated with luspatercept achieved Hb > 10.0 g/dL, from 55.2% (n = 53/96) at Week 12, 63.0% (n = 58/92) at Week 24, 67.0% (n = 61/91) at Week 36, to 69.7% (n = 62/89) at Week 48 (Figure 1). A ≥ 1.0 g/dL increase from baseline in mean Hb level during any rolling 24-week period was achieved by 68.4% (n = 13/19) of patients treated with luspatercept with baseline Hb < 7.0 g/dL, 88.9% (n = 32/36) of those with baseline Hb ≥ 7.0 and ≤ 8.5 g/dL, and 87.8% (n = 36/41) of those with baseline Hb > 8.5 g/dL.
Conclusions: This analysis of the BEYOND clinical trial showed the majority of patients with NTDT receiving luspatercept achieved Hb > 10.0 g/dL over time, a threshold that is consistently associated with reduced morbidity and mortality in the literature. Clinically relevant Hb increases of ≥ 1.0 g/dL were observed across all baseline Hb levels. These findings further support the ability of luspatercept to achieve clinically meaningful increases in Hb for the majority of patients with NTDT, irrespective of baseline severity of anemia.
Disclosures
Musallam:Novartis: Consultancy; CRISPR Therapeutics: Consultancy; Vifor Pharma: Consultancy; Pharmacosmos: Consultancy; Agios Pharmaceuticals: Consultancy; Celgene Corp (Bristol Myers Squibb): Consultancy. Taher:Bristol Myers Squibb: Consultancy, Research Funding; Ionis Pharmaceuticals: Consultancy, Research Funding; Vifor Pharma: Consultancy, Research Funding; Imara: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Porter:Celgene: Consultancy, Honoraria; La Jolla Pharmaceuticals: Honoraria; Protagonism: Honoraria; Silence Therapeutics: Honoraria; Agios: Consultancy, Honoraria; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; VIFOR: Honoraria; bluebird bio: Consultancy, Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kattamis:Chiesi: Honoraria; Novartis: Consultancy, Honoraria, Research Funding; ViFOR: Consultancy; IONIS: Consultancy; VERTEX: Consultancy, Honoraria; AMGEN: Consultancy; AGIOS Pharmaceuticals: Consultancy; BMS/Celgene: Consultancy, Honoraria, Research Funding. Glassberg:Novartis: Ended employment in the past 24 months; BMS: Current Employment, Current holder of stock options in a privately-held company. Bueno:BMS: Current Employment. Shetty:Celgene/Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Lersch:BMS: Current Employment, Current holder of stock options in a privately-held company. Rosettani:Bristol-Myers Squibb: Current Employment. Cappellini:Sanofi Genzyme: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Vertex: Membership on an entity's Board of Directors or advisory committees; Agios: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Silence: Membership on an entity's Board of Directors or advisory committees; BMS: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal